Atomic Structure and Atomic Models
Atom: is a particle of matter consists of a central nucleus is positively charged and contains one or more relatively heavy particles known as protons and neutrons surrounded by one or more electrons (negatively charged).

We have four different atomic models:
1- Dalton’s Model
-
Dalton based his theory on the law of conservation of mass and the law of constant composition.
-
The first part of his theory states that all matter is made of atoms, which are indivisible.
-
The second part of the theory says all atoms of a given element are identical in mass and properties.
-
The third part says compounds are combinations of two or more different types of atoms.
-
The fourth part of the theory states that a chemical reaction is a rearrangement of atoms.
2- Thomson’s Model (plum pudding model)
-
J.J. Thomson’s experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons.
-
Thomson’s plum pudding model of the atom had negatively charged electrons embedded within a positively charged “soup.”


3- Rutherford’s Model
Rutherford, in his experiment, directed high energy streams of α-particles from a radioactive source at a thin sheet (100 nm thickness) of gold. In order to study the deflection caused to the α-particles.

Observations of Rutherford’s Alpha Scattering Experiment
- A major fraction of the α-particles bombarded towards the gold sheet passed through the sheet without any deflection, and hence most of the space in an atom is empty.
- Some of the α-particles were deflected by the gold sheet by very small angles, and hence the positive charge in an atom is not uniformly distributed. The positive charge in an atom is concentrated in a very small volume.
- Very few of the α-particles were deflected back, that is only a few α-particles had nearly 180o angle of deflection. So the volume occupied by the positively charged particles in an atom is very small as compared to the total volume of an atom.
4- Bohrs Model
Postulates of Bohr’s Model of an Atom
- In an atom, electrons (negatively charged) revolve around the positively charged nucleus in a definite circular path called orbits or shells.
- Each orbit or shell has a fixed energy and these circular orbits are known as orbital shells.
- The energy levels are represented by an integer (n=1, 2, 3…) known as the quantum number. This range of quantum number starts from nucleus side with n=1 having the lowest energy level.
- The electrons in an atom move from a lower energy level to a higher energy level by gaining the required energy and an electron moves from a higher energy level to lower energy level by losing energy.

Important terms:
Absorption: When electron absorb energy from (photon) it will be excited from ground state to excited state.
Emission: When electron loses energy, it will be drops from excited state to ground state.
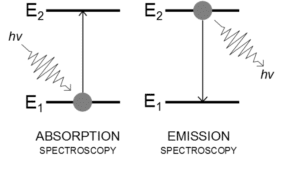
When electron move from ground state to outside the atom this process called ionization (losing of electrons).

Subatomic Particles
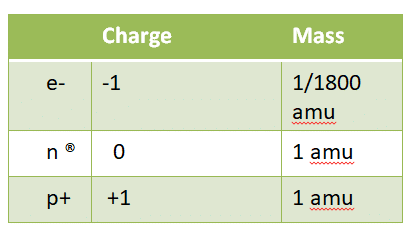 We know that atoms consist of nucleus that contain (proton & neutron), and electrons.
We know that atoms consist of nucleus that contain (proton & neutron), and electrons. - amu: atomic mass unit=1/2 of carbon atom mass.
- Atomic number: the number of proton (p+) in the atom, so each atom, (element) has its own atomic number.
- For neutral elements atomic number equal number of protons, equal number of electrons.
- The mass number (atomic number) equal number of protons + number of neutrons.
Example
22.99Na11
- Note that: 11 Refer to atomic number
- 22.990: Refer to mass number
- So in sodium atom (Na) there are
- 11 Proton
- 11 Electron
- 23-11=12 neutron
Ions
- If atom gain electron, anion will be form (- ion)
- If atom loses electron , cation will be form (+ ion)
- In sodium (Na) we have 11 p+ , 12 n, 10 e- (11-1) , charge(+1)
- In oxygen (o) we have 8 p+ , 8 n , 10 e- (8+2) , charge(-2)
- In sulfur (S) we have 16 p+ , 16 n , 18 e- (16+2) , charge (-2)
- In aluminum(Al) we have 13 p+, 14 n , 10 e- (13-3) , charge(+3)



Isoelectronic particles
Refers to two atoms, ions or molecules that have the same electron configuration and the same number of valence electrons.
Example: Na+1, O-2 , Al+3, Ne, N-3
Isotopes
Atoms of the same element (or have the same atomic number or have the same number of proton) but have different atomic mass (mass number, or number of neutrons)
H atom as show in the below figure.

How to find the average atomic mass of an element?
Example

Element X has three isotopes
Calculate average atomic mass?
Average atomic mass of X
Solution
(20*80/100) + (21*15/100) + (22*5/100) =
16 + 3.15 + 1.1 =20.25
