Mendeleev’s and Moseley Periodic Table Arrangement.
-
- The periodic table (also known as the periodic table of elements) is organized so scientists can quickly discern the properties of individual elements such as their mass, electron number, electron configuration and their unique chemical properties.
- Metals reside on the left side of the table, while non-metals reside on the right.
- Organizing the elements to help further our understanding was first provided by Dmitri Mendeleev.
- Periodic trends are Specific patterns that are present in the periodic table that illustrate different aspects of a certain element, including its size and its electronic properties.
- Major periodic trends include: electronegativity , ionization energy, electron affinity, atomic radius, melting point, and metallic character.
- Periodic trends, arising from the arrangement of the periodic table, provide chemists with an invaluable tool to quickly predict an element’s properties.
- These trends exist because of the similar atomic structure of the elements within their respective group families or periods, and because of the periodic nature of the elements.
Mendeleev’s and Moseley periodic table arrangement.
- Mendeleev’s Periodic table, the elements were arranged in the increasing order of their atomic masses.
- Only 63 elements were discovered during Mendeleev’s time, so he made the arrangement of those 63 elements in his table

Figure 1.1: Mendeleev Periodic Table - His table contains some gaps (undiscovered elements)
- He predicted the discovery The physical properties of Sc, Ga, Ge.
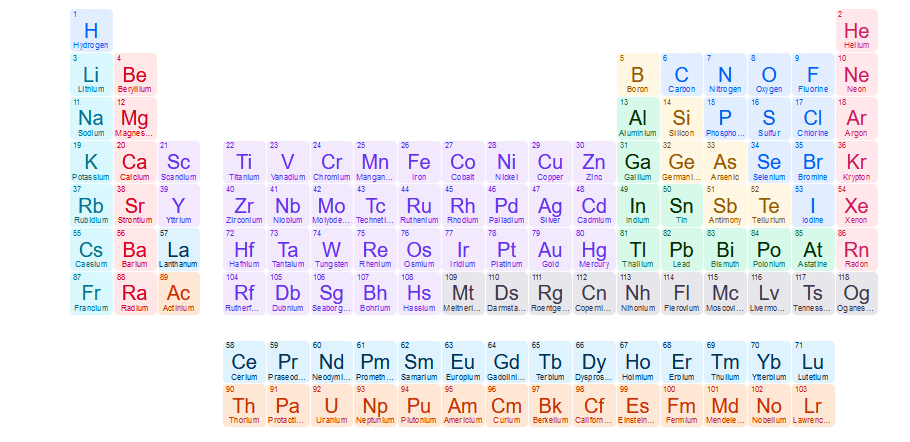
Figure 1.2: Modern Periodic Table(Moosely) - Moseley periodic table is a tabular display of the chemical elements where they are arranged in order of increasing atomic number.
- Moseley’s Periodic table gave us the clear concept for the arrangement of elements. And because of his valuable efforts, today we have a complete periodic table with 118 elements without any limitations in arrangement.
Difference between Mendeleev and Moseley Periodic table
Mendeleev’s Periodic table Moseley’s Periodic table Arrangement of elements is based on atomic mass. Arrangement of elements is based on atomic number Only 63 elements were present in Mendeleev’s Periodic table in 1869. Around 92 elements were present in Moseley’s Periodic table in 1914. It has 8 groups & 6 periods. It has 18 groups & 7 periods Classification was based on hydrides and oxides formed by the chemical reaction Mendeleev did not explain why elements of same groups have similar chemical properties, and elements of same periods have different properties. Noble gases are not included in Mendeleev’s periodic table No positions for isotopes in Mendeleev’s table. Classification was based on electron configuration. Moseley explained the reason behind the similar properties of elements in groups, and the reason was electron configuration. Noble gases are included in the Moseley Periodic table (in group 18). The isotopes have same position along with the neutral atom
