Introduction to Biochemistry
- The Ground state: when electrons are in positions of lowest energy possible (normal).
- Excited state: when electrons are in a temporary position of higher energy than ground state, and this is a very unstable state in which the electrons quickly return to the ground state releasing their extra energy. This energy will be used in reactions. (ETC in Respiration and photosynthesis).
- Element: Any substance that contains one kind of atom.
- Isotopes: Elements with the same number of protons but different number of neutrons.
- Some isotopes decay at a known rate of time (Half-life) ⇒ measure the age of fossils and rocks.
- Some isotopes are radioactive ⇒ used as tracers.
- Compound: Consists of atoms of two or more different elements.
- Mixture: Consists of two or more different elements / compounds.
**********************************************
Types of Chemical Bonding
At the formation of any bond:
- Energy must be supplied to break a bond ⇒ then: some energy must be released to form new stable bonds. “Will be discussed in Unit 3”.
- Types of Chemical Interactions:
- Covalent Bond:
- Sharing electrons.
- Can be polar or nonpolar.
- Ionic Bond:
- Losing and gaining electrons.
- All polar.
- Hydrogen Bond:
- Mostly between polar molecules.
- Hydrophobic Interactions:
- When nonpolar repel from polar they tend to bind together away from the polar side.
- Covalent Bond:

- Types of Chemical Compounds:
- Hydrophobic: Water repelling; non-polar molecules (not attracted to water) e.g. Fats and Oils.
- Mostly covalent.
- Hydrophilic: Water loving; polar molecules (attracted to water) e.g. ions and alcohols.
- Ionic and some covalent.
- Amphipathic: Both sides (polar and non-polar).
- Mostly covalent.
- Hydrophobic: Water repelling; non-polar molecules (not attracted to water) e.g. Fats and Oils.
*** Like – dissolves – Like ***
(Polar attracts to polar)
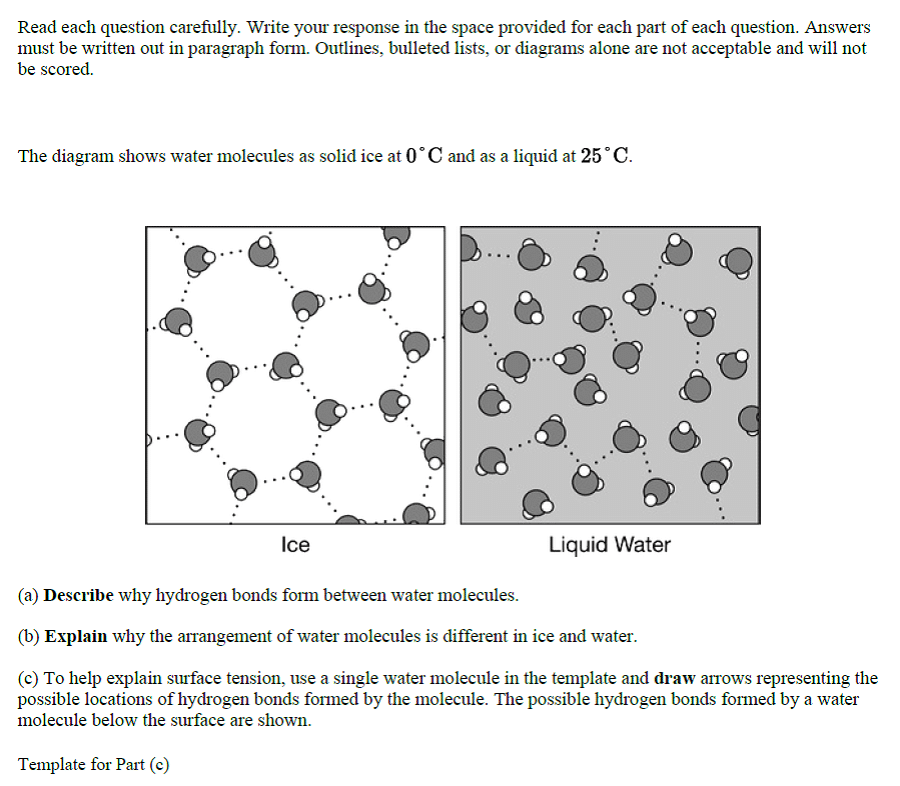
Characteristics of Water:
- Water is a small and polar molecule.
- There are strong forces of attraction between the water molecules which determine their distinctive property.


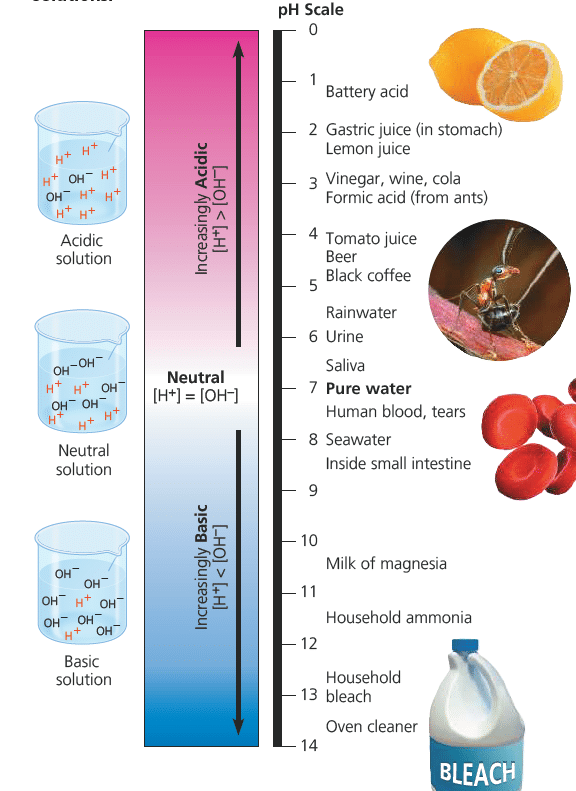
The relationship between H+ and pH:
- When the H+ concentration is 1×10-5 we say that the pH is 5.
- pH 7 is neutral (concentrations of H+ & OH– are equal)
- The higher the pH, the less H+ concentration, the more OH– concentration.
pH = – log[H+]
- When the pH changes from
- 2 to 4 ⇒ H+ ion concentration decreases by 100 times.
- 8 to 3 ⇒ H+ ion concentration increases by 100000 times.
- Buffers: are solutions which can resist changes in pH; E.g. Bicarbonate buffer.
H+ + HCO3– ⇒ H2CO3 ⇒ H2O + CO2
- The importance of water in Life
- Most of the water is in its liquid state:
- Important for many metabolic processes.
- Stable environment for aquatic organisms.
- When it evaporates it cools bodies down.
- Many substances dissolve in water providing ions and minerals for the aquatic organisms.
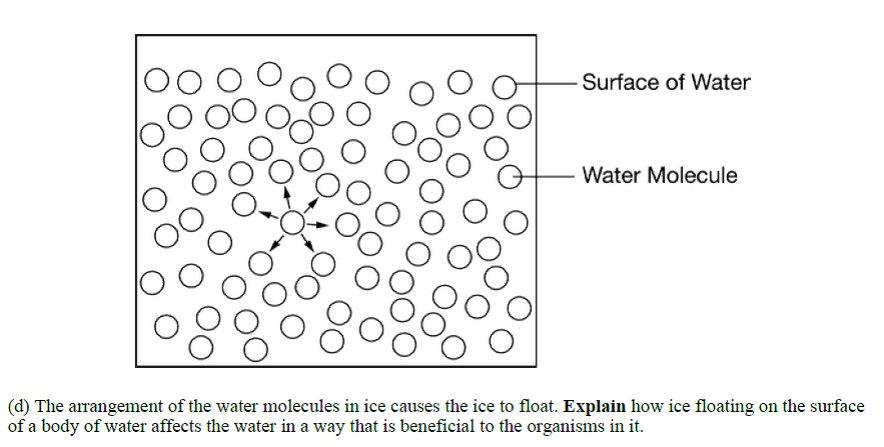
- Floating ice acts as an insulator to maintain a stable aquatic environment.
- Transparent for more photosynthesis.
- Water can help in controlling the pH as H+ donor or a receiver.
- Most of the water is in its liquid state:
Questions:
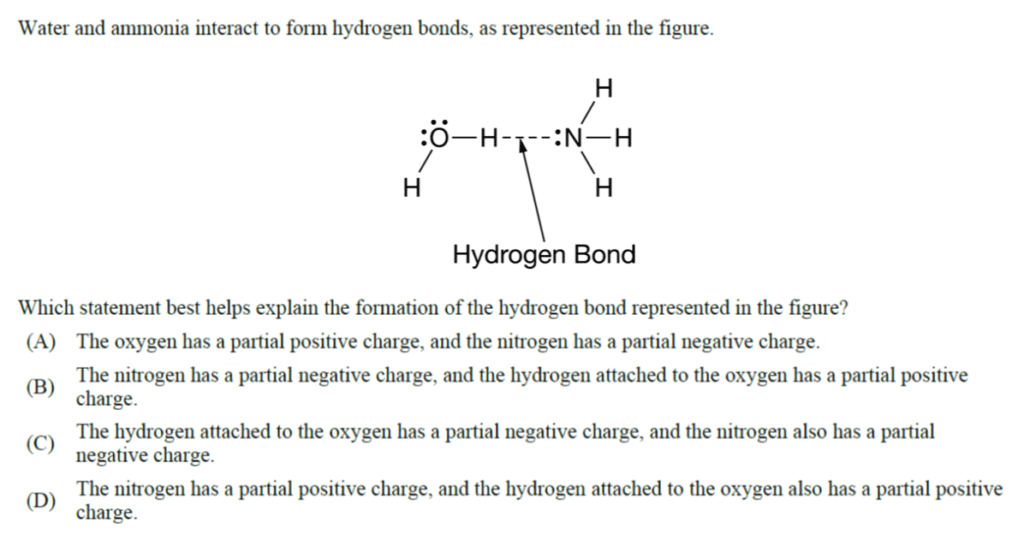
1. B
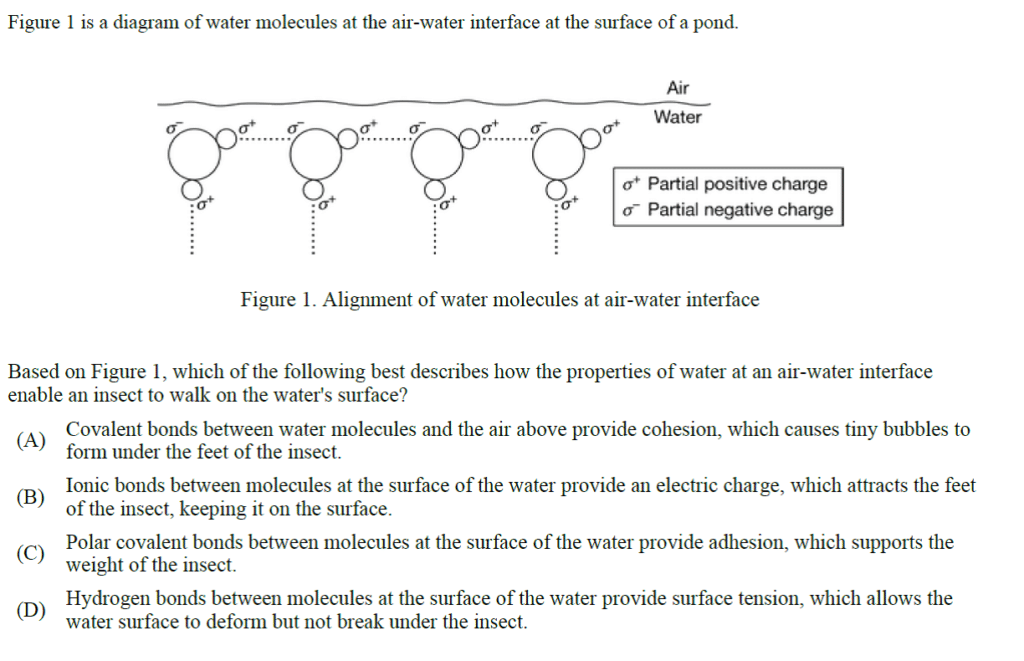
2. D
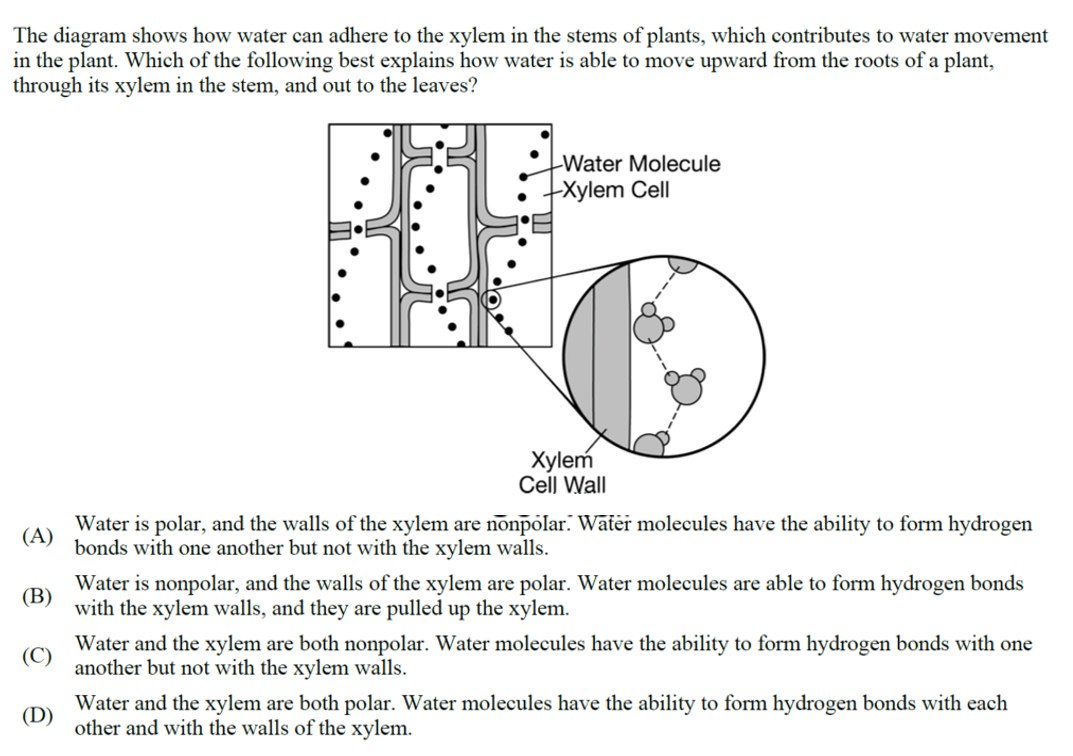
3. D
 Homework Practice:
Homework Practice:
Question One:
Question Two Scoring Guidelines

